Goaltide Daily Current Affairs 2024
Current Affair 1:
Uniform Code of Pharmaceutical Marketing Practices (UCPMP)
News:
The Department of Pharmaceuticals issued the Uniform Code for Pharmaceutical Marketing Practices (UCPMP) 2024 on March 13, specifying the rules of the use of the words “safe’’ and “new’’ for drugs, and stated that medical representatives must not employ any inducement or subterfuge to gain an interview, and that they must not pay, under any guise, for access to a healthcare professional.

The Government has put in place a Uniform Code for Pharmaceutical Marketing Practices (UCPMP) for pharmaceutical companies, which is in operation since 01.01.2015, to prevent unethical practices by the pharmaceutical companies.
- This code governs the conduct of pharmaceutical companies in their marketing practices, duly covering the various aspects such as medical representatives, textual and audio-visual promotional materials, samples, gifts, etc.
- Further, the code establishes relationship with healthcare professionals, wherein the provisions related to travel facilities, hospitality and cash or monetary grants to physicians or their families have been elaborated.
- The code also details the mode of operation of the code, responsibilities of the Pharmaceutical Associations in constituting the Ethics Committee for Pharmaceutical Marketing Practices (ECPMP) for handling the complaints and Apex Ethics Committee for Pharmaceutical Marketing Practices (AECPMP)
- The code has been adopted by the all the major associations of pharmaceutical companies and the Department on various instances has reviewed implementation of the code by the pharmaceutical’s associations.
Besides UCPMP, there exists sufficient and enforceable legal regime to counter, control and dis-incentivize the unethical marketing practices such as “Indian Medical Council Professional Conduct, Etiquette and Ethics) Regulations, 2002” under the Indian Medical Council Act, 1956, provisions available under Income Tax Act, Drugs and Cosmetics Act, Prevention of Corruption Act, etc.
Something more:
- As per information received from Central Drugs Standard Control Organisation (CDSCO) under the Ministry of Health & Family Welfare that the manufacture, sale and distribution of drugs in the country are regulated under the provisions of Drugs & Cosmetics Act, 1940.
- Licenses for manufacture, sale and distribution of drugs are granted by the State Licensing Authorities (SLAs) appointed by respective State Governments.
- Under the said Rules, Fixed Dose Combinations (FDC) is a New Drug. For the manufacture of any FDC falling under the definition of New Drug, permission is required from Central Drugs Standard Control Organisation (CDSCO) before obtaining manufacturing license for the New Drug from the concerned State Licensing Authority.
- Under the aforesaid Act, manufacture/sale/distribution of any banned drug is a punishable offence. State Licensing Authorities are empowered to act in this regard.
Current Affair 2:
Prasar Bharati (Broadcasting Corporation of India)
News:
In a significant development for the Indian media landscape, public broadcaster Prasar Bharati launched PB-SHABD, a news-sharing service designed to provide daily news feeds in various formats to media organizations.
About:
It is a statutory autonomous body set up by an Act of Parliament (Prasar Bharati (Broadcasting Corporation of India) Act, 1990).
It comprises the Doordarshan Television broadcasting and Akashvani (formerly All India Radio AIR), which were earlier media units of the Ministry of Information and Broadcasting.
The Act grants autonomy to All India Radio and to Doordarshan, both of which were previously under government control.
The headquarters of the Corporation shall be at New Delhi and the Corporation may establish offices, with the previous approval of the Central Government, outside India.
About Prasar Bharati Board.
The general superintendence, direction and management of the affairs of Prasar Bharati are vested in the Prasar Bharati Board, which is headed by the Chairman (NOT A MINISTER). The Chairman is a Part Time Member with a three-year tenure, subject to the age limit of 70 years.
The Prasar Bharati Board meets at least 6 times in a year and the meetings are presided over by the Chairman.
By the Prasar Bharati Act, all property, assets, debts, liabilities, payments of money due, as well as all suits and legal proceedings involving Akashvani (All India Radio) and Doordarshan were transferred to Prasar Bharati.
Current Affair 3:
Signals Technology Evaluation and Adaptation Group (STEAG)
News:

In a significant step towards preparing for the future of warfare, the Indian Army has established the “Signals Technology Evaluation and Adaptation Group” (STEAG), a first-of-its-kind specialised technology unit.
The primary objective of STEAG is to conduct research and evaluation of cutting-edge communication technologies such as Artificial Intelligence (AI), 5G and 6G networks, machine learning, and quantum technologies for defence applications.
Current Affair 4:
Avaana Sustainability Fund (ASF)
The Small Industries Development Bank of India (SIDBI) has achieved a significant milestone by securing approval from the Green Climate Fund (GCF) for its first anchored project, the Avaana Sustainability Fund (ASF).

About SIDBI:
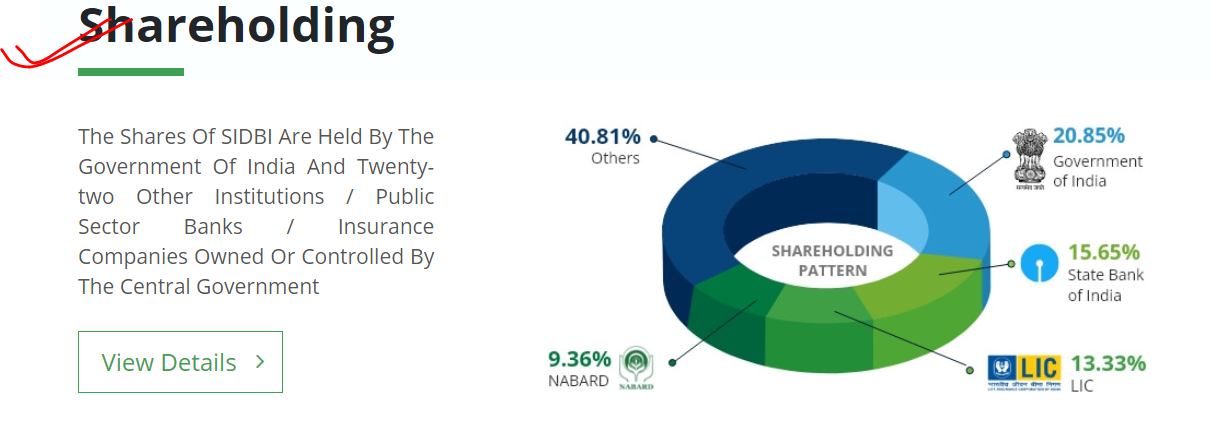
Small Industries Development Bank of India (SIDBI) set up on 2nd April 1990 under an Act of Indian Parliament, acts as the Principal Financial Institution for Promotion, Financing and Development of the Micro, Small and Medium Enterprise (MSME) sector as well as for co-ordination of functions of institutions engaged in similar activities.

<< Previous Next >>



